Mucosal barrier tissues, comprising a layer of tightly-bonded epithelial cells in intimate molecular communication with an underlying matrix-rich stroma containing fibroblasts and immune cells, are outstanding targets for medication in opposition to an infection, power irritation, and different illness processes.
Although human in vitro fashions of such obstacles are wanted for mechanistic research and drug growth, variations in extracellular matrix (ECM) wants of epithelial and stromal cells hinder efforts to create such fashions.
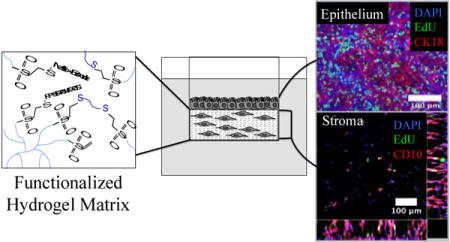
Here, utilizing the endometrium for example mucosal barrier, we describe a synthetic, modular ECM hydrogel appropriate for 3D practical co-culture, that includes elements that may be transformed by cells and that reply dynamically to sequester native cell-secreted ECM attribute of every cell kind.
The synthetic hydrogel combines peptides with off-the-shelf reagents and is thus accessible to cell biology labs. Specifically, we first recognized a single peptide as appropriate for preliminary attachment of each endometrial epithelial and stromal cells utilizing a 2D semi-empirical display screen.
Then, utilizing a co-culture system of epithelial cells cultured on prime of gel-encapsulated stromal cells, we present that inclusion of ECM-binding peptides within the hydrogel, together with the integrin-binding peptide, results in enhanced accumulation of basement membrane beneath the epithelial layer and extra fibrillar collagen matrix meeting by stromal cells over two weeks in tradition.
Importantly, endometrial co-cultures composed of both cell traces or main cells displayed hormone-mediated differentiation as assessed by morphological adjustments and secretory protein manufacturing.
A multiplex evaluation of apical cytokine and progress issue secretion evaluating cell traces and main cells revealed strikingly completely different patterns, underscoring the significance of utilizing main cell fashions in evaluation of cell-cell communication networks. In abstract, we outline a “one-size-fits-all” synthetic ECM that enables long-term, physiologically responsive co-cultures of epithelial and stromal cells in a mucosal barrier format.
In vivo cloning of as much as 16 kb plasmids in E. coli is so simple as PCR.
The exact meeting of outlined DNA sequences into plasmids is a vital process in bioscience analysis. While a quantity of molecular cloning methods have been developed, many strategies require specialised costly reagents or laborious experimental process.
Not surprisingly, standard cloning methods based mostly on restriction digestion and ligation are nonetheless generally utilized in routine DNA cloning. Here, we describe a easy, quick, and economical cloning methodology based mostly on RecA- and RecET-independent in vivo recombination of DNA fragments with overlapping ends utilizing E. coli.
All DNA fragments had been ready by a 2-consecutive PCR process with Q5 DNA polymerase and used immediately for transformation leading to 95% cloning accuracy and zero background from parental template plasmids. Quantitative relationships had been established between cloning effectivity and three factors-the size of overlapping nucleotides, the quantity of DNA fragments, and the dimensions of goal plasmids-which can present basic steering for choosing in vivo cloning parameters.
The methodology could also be used to precisely assemble as much as 5 DNA fragments with 25 nt overlapping ends into comparatively small plasmids, and three DNA fragments into plasmids as much as 16 kb in measurement.
The complete cloning process could also be accomplished inside 2 days by a researcher with little coaching in cloning. The mixture of excessive accuracy and zero background eliminates the necessity for screening a big quantity of colonies.
The methodology requires no enzymes apart from Q5 DNA polymerase, has no sequence restriction, is extremely dependable, and represents one of the best, quickest, and least expensive cloning methods available.
Our methodology is especially appropriate for widespread cloning duties within the lab the place the first purpose is to shortly generate a plasmid with a pre-defined sequence at low prices.
