Biochemistry, study of the chemical substances and processes that occur in plants, animals, and microorganisms and the changes they undergo during development and life. It deals with the chemistry of life, and as such draws on the techniques of analytical, organic, and physical chemistry, as well as those of physiologists interested in the molecular basis of life processes. All chemical changes within the organism, whether it be the breakdown of substances, usually to obtain the necessary energy or the accumulation of complex molecules necessary for life processes, are collectively called metabolism.
These chemical changes depend on the action of organic catalysts known as enzymes, and enzymes, in turn, depend on their existence on the genetic apparatus of the cell. It is not surprising, therefore, that biochemistry enters the investigation of chemical changes in disease, drug action, and other aspects of medicine, as well as nutrition, genetics, and agriculture.
The term biochemistry is synonymous with two somewhat older terms: physiological chemistry and biological chemistry. Aspects of biochemistry that deal with the chemistry and function of very large molecules (eg, proteins and nucleic acids) are often grouped under the term molecular biology. Biochemistry is a young science, known under that term only since around 1900. However, its origins go back much further; its early history is part of the early history of both physiology and chemistry.
Study areas
A description of life at the molecular level includes a description of all the complexly interrelated chemical changes that occur within the cell, that is, the processes known as intermediary metabolism. The processes of growth, reproduction, and heredity, also subjects of biochemists’ curiosity, are intimately related to intermediary metabolism and cannot be understood independently of it. The properties and capabilities exhibited by a complex multicellular organism can be reduced to the properties of the individual cells of that organism, and the behaviour of each individual cell can be understood in terms of its chemical structure and the chemical changes that occur within that cell.
Chemical composition of living matter.
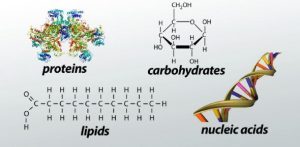
Every living cell contains, in addition to water and salts or minerals, a large number of organic compounds, substances composed of carbon combined with varying amounts of hydrogen and, usually, oxygen as well. Nitrogen, phosphorus, and sulfur are also common constituents. In general, most of the organic matter in a cell can be classified as (1) protein, (2) carbohydrate, and (3) fat or lipid. Nucleic acids and various other organic derivatives are also important constituents. Each class contains a great diversity of individual compounds. There are also many substances that cannot be classified in any of the above categories, although usually not in large quantities.
Proteins are essential to life, not only as structural elements (eg, collagen) and to provide a defence (as antibodies) against invading destructive forces, but also because the essential biocatalysts are proteins. The chemistry of proteins is based on the research of the German chemist Emil Fischer, whose 1882 work showed that proteins are very large molecules, or polymers, made up of about 24 amino acids. Proteins can range in size from small (insulin with a molecular weight of 5,700 (based on the weight of a hydrogen atom as 1)) to very large molecules with molecular weights of over 1,000,000.
The first complete amino acid sequence was determined for the insulin molecule in the 1950s. By 1963 the amino acid chain in the protein enzyme ribonuclease (molecular weight 12,700) had also been determined, with the help of the powerful physical techniques of analysis. of X-ray diffraction. In the 1960s, Nobel Prize winners JC Kendrew and M. F. Perutz, using X-ray studies, built detailed atomic models of the proteins haemoglobin and myoglobin (the respiratory pigment in muscle), which were later confirmed by sophisticated chemical studies. The continuing interest of biochemists in protein structure is based on the fact that the arrangement of chemical groups in space provides important clues about the biological activity of molecules.
Carbohydrates include substances such as sugars, starch, and cellulose. The second quarter of the 20th century saw a startling advance in understanding how living cells handle small molecules, including carbohydrates. Carbohydrate metabolism became elucidated during this period, and the elaborate pathways of carbohydrate breakdown and subsequent storage and utilization were gradually described in terms of cycles (eg, the Embden-Meyerhof glycolytic cycle and the Krebs cycle). ). The involvement of carbohydrates in respiration and muscle contraction was well elaborated in the 1950s. Refinements of the schemes continue.
Fats, or lipids, are a heterogeneous group of organic chemicals that can be extracted from biological material by nonpolar solvents such as ethanol, ether, and benzene. The classic work on the formation of body fat from carbohydrates was done in the early 1850s. Those studies, and subsequent confirmatory evidence, have shown that the conversion of carbohydrates to fat occurs continuously in the body. The liver is the main site of fat metabolism.
The absorption of fat in the intestine was studied as early as the 1930s. It is known that the control of fat absorption depends on a combined action of the secretions of the pancreas and bile salts. Abnormalities of fat metabolism, which give rise to disorders such as obesity and rare clinical conditions, are the subject of much biochemical research. Equally interesting to biochemists is the association between high levels of fat in the blood and the development of arteriosclerosis (“hardening” of the arteries).
Nucleic acids are large, complex compounds of very high molecular weight present in the cells of all organisms and in viruses. They are of great importance in the synthesis of proteins and in the transmission of hereditary information from one generation to the next. Originally discovered as components of cell nuclei (hence their name), it was assumed for many years after their isolation in 1869 that they were found nowhere else. This assumption was not seriously questioned until the 1940s, when it was determined that there are two types of nucleic acid: deoxyribonucleic acid (DNA), in the nuclei of all cells and in some viruses; and ribonucleic acid (RNA), in the cytoplasm of all cells and in most viruses.
The profound biological importance of nucleic acids gradually came to light during the 1940s and 1950s. Attention turned to the mechanism by which protein synthesis and genetic transmission were controlled by nucleic acids (see below, Genes). During the 1960s, experiments were aimed at refining the genetic code. Promising attempts were made in the late 1960s and early 1970s to replicate nucleic acid molecules outside the cell, that is, in the laboratory. By the mid-1980s, genetic engineering techniques had achieved, among other things, in vitro fertilization and DNA recombination (so-called gene splicing).
Evolution and origin of life.
Space exploration beginning in the mid-20th century intensified speculation about the possibility of life on other planets. At the same time, the man was beginning to understand some of the intimate chemical mechanisms used for the transmission of hereditary characteristics. By studying the structure of proteins in different species, it was possible to see how the amino acid sequences of functional proteins (for example, haemoglobin and cytochrome) have been altered during phylogeny (the development of species). It was natural, therefore, for biochemists to regard the problem of the origin of life as a practical one. The synthesis of a living cell from inanimate material was not considered an impossible task for the future.