OBJECTIVETrimethylamine-N-oxide (TMAO) produced by intestine microbiota metabolism of dietary choline and carnitine has been proven to be related to elevated threat of heart problems (CVD) and to offer incremental clinical prognostic utility past conventional threat elements for assessing a affected person’s CVD threat.
The purpose of this research was to develop an automatic nuclear magnetic resonance (NMR) spectroscopy assay for quantification of TMAO focus in serum and plasma utilizing a high-throughput NMR clinical analyzer.
METHODSKey steps in assay improvement included:
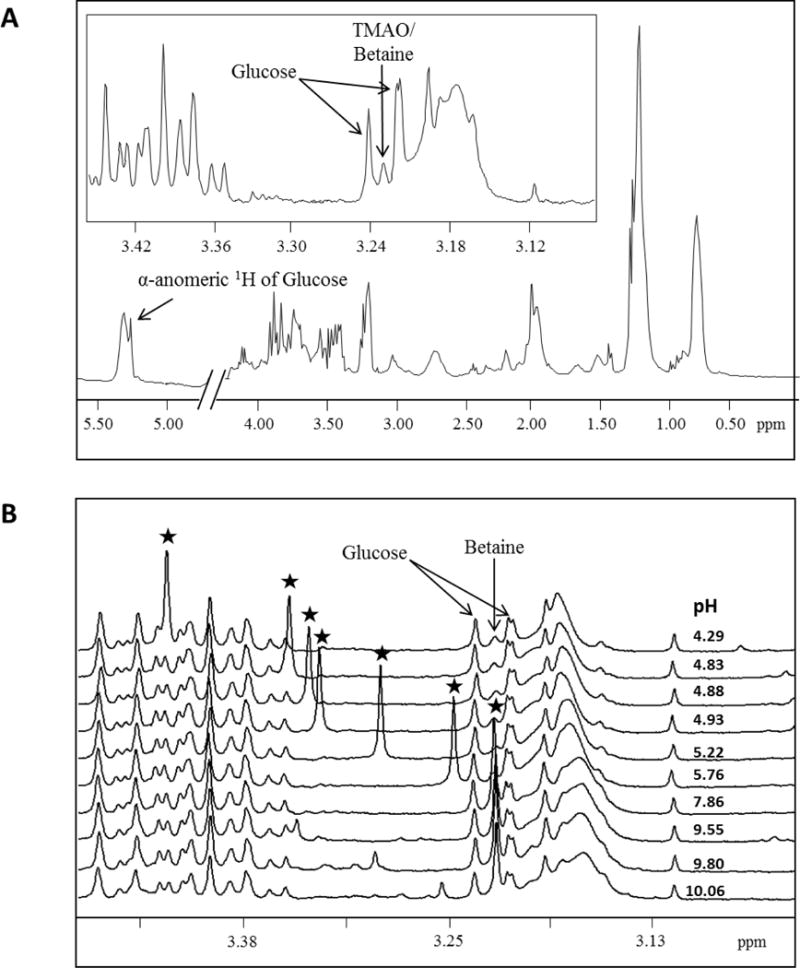
(i) shifting the TMAO analyte peak to a much less crowded area of the spectrum with a pH buffer/reagent, (ii) attenuating the broad protein background sign in the spectrum and
(iii) utilizing a non-negative least squares algorithm for peak deconvolution. Assay efficiency was evaluated in keeping with Clinical and Laboratory Standards Institute pointers.
A way comparability research was carried out to check TMAO concentrations quantified by NMR and mass spectrometry (MS).RESULTSThe within-run and within-lab imprecision ranged from 4.Three to 14.5%. Under the acquisition technique employed, the NMR assay had a restrict of clean, detection and quantitation of 1.6, 3.0 and 3.3μM, respectively. Linearity was demonstrated inside the reportable vary of 3.Three to 3000μM.
TMAO measurements utilizing the NMR assay, which entails minimal pattern preparation, in contrast effectively with values obtained with the MS-based assay (R2=0.98).CONCLUSIONSThe NMR primarily based assay offers a easy and correct measurement of circulating TMAO ranges amenable to the high-throughput calls for of the clinical chemistry laboratory.
Moreover, assay efficiency allows the ranges of TMAO to be quantified in serum or plasma at clinically actionable concentrations for the evaluation of heart problems dangers and individualized dietary monitoring.
MAPS-seq: magnetic bead-assisted parallel single-cell gene expression profiling.
Recently developed single-cell RNA sequencing strategies enable the simultaneous profiling of the transcriptomes of hundreds of particular person cells.
However, present strategies nonetheless require superior tools or entail substantial waste of reagents. Here, we introduce magnetic bead-assisted parallel single-cell gene expression sequencing (MAPS-seq), a microwell-based technique that swimming pools samples earlier than the reverse transcription step, rising the ease of pattern preparation and lowering reagent waste.
Moreover, as a result of this technique makes use of common reagents and normal molecular biology lab devices, it’s straightforward to implement, even in labs that haven’t beforehand carried out single-cell RNA sequencing.
We validated our technique by demonstrating that it may well generate gene expression information at the single-cell stage. We then utilized the MAPS-seq technique to investigate 237 human myelogenous leukemia cells handled with one of three totally different medication or dimethyl sulfoxide.
We noticed transcriptional modifications and recognized marker genes that point out a drug response. Furthermore, the MAPS-seq technique produced information of comparable high quality to these of present single-cell RNA sequencing strategies.
Consequently, we anticipate that our technique will present researchers with a extra accessible, much less wasteful, and much less burdensome technique for investigating the transcriptomes of particular person cells.
